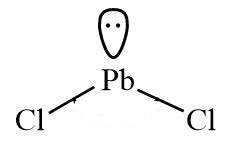
Q-what is the molecular geometry of Pbcl2 ?
Ans- Molecular geometry -arrangement of atoms in relation to a central atom in three-dimension space.
Molecular geometry of pbcl2 is bent or angular or v shape.
Explanation-To know any given compounds molecular geometry first we find its hybridization .
Given compound is PbCl2 ( lead chloride) we calculate the hybridization of any compound by using this formula.
Z=1/2(velence electron of central atom+(-ve)-(+ve)+no. of monovalent atom)
Where Z= sum of bond pair and lone pair.
In periodic table lead is in 14th group .It has 4Valence electrons. There is 2 Cl atom with lead, so number of monovalent atom is 2&no (-ve)charge,no(+ve) charge.
Z=1/2(4+2)
=3
If Z =3 then hybridization is Sp²(VSPER theory). The molecular geometry of Pbcl2 is bent or angular or v shaped.
M.Sc(chem),B.Ed


That's a beautiful explanation of molecular geometry of pbcl2
ReplyDeleteIt's perfect
ReplyDeletePost a Comment